Neurons are computers: The Computational Power of Dendrites
The human brain is composed of billions of computational units, called neurons. Neurons transmit information to each other through electrical pulses, or spikes. A common misrepresentation is that the neuron is the basic conputational building block; that spikes are transferred and integrated at the soma, and that axons and dendrites are simply a network of electrical cables connecting the neurons together. Treating the dendrite as cabling is convenient; the mathematics are simpler, and it’s easier to reason about the role of neurons, both individually and in groups. As we’ll see, this simplification is wrong, and it glosses over a critical computational component in our neural architecture.
If you missed it, please check out my first post: Efficiency is Key: Lessons from the Human Brain
Some of the graphics and animations below were created in Julia with Plots.jl. Check out the IJulia notebook.
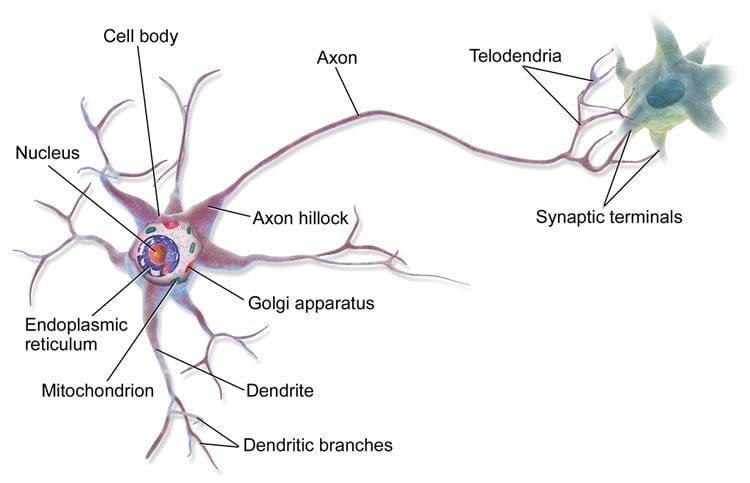
Figure A: Components of a Neuron [1]
The Basics of Spiking
A neuron’s output is a sequence of electrical bursts; termed action potentials, or spikes. A neuron’s cell body (soma) accumulates post-synaptic (after the synaptic cleft) chemical energy in the form of a membrane potential. At the site of the axon hilcock, net input potentials are summated, and a spike is generated. This spike travels quickly through short sections of the axon surrounded by myelin sheath, where the signal is then boosted at each node (the gaps between sheaths). This successive boosting allows the current to quickly reach the tips of the axonal branches.
Axons (output) form connections with the dendrites (input) of other neurons; this connection is called a synapse. When a signal reaches the end of an axon at a synapse, it triggers the release of neurotransmitters which then cross the synaptic gap, binding and opening channels on the dendrite. The transfer of chemicals (for example sodium and potassium) are what change the interal charge of the dendrite, and also the soma. This charge builds up until crossing a theoretical membrane threshold and triggering a run-away process which results in a spike. (See this short video for a visual explanation of the process)
Axons terminate at many pre-synaptic (before the synaptic cleft) sites. Synaptic terminals are possible in many configurations. Axons can synapse directly with the soma (axosomatic), leading to direct potential transfer, or they could even synapse with another synaptic terminal (axosynaptic), leading to modulatory effects. However the most common connection is axon-to-dendrite (axodendritic).

Figure B: Synaptic Connection Locations [1]
Location, location, location…
The location of the synaptic terminal has a large effect on the resulting change in membrane potential (which, when high enough, will produce a spike). Those synapses connecting axon to soma (axosomatic) have nearly direct energy transfer; the postsynaptic response (i.e. how the receiving neuron responds) will be somewhat linear when compared with the presynaptic input.
For synapses on the dendritic tree, the postsynaptic potential (PSP) is affected both by passive properties of dendrites [2], and synaptic inhibition from neighboring synapses [3], both driving a sublinear response. This means that postsynapitic potentials have diminishing marginal response as more synapses on the dendritic segment are active.
In addition, dendritic synapses have active properties. When synapses are active in isolation, the relative response at the soma is generally small. However, when multiple synapses become active with spatio-temporal proximity (meaning nearby in both space and time) then it can trigger a supralinear response: a dendritic spike. This active conductance is relatively large as compared to the individial postsynaptic responses; however it quickly saturates, and additional presynaptic input has little effect.
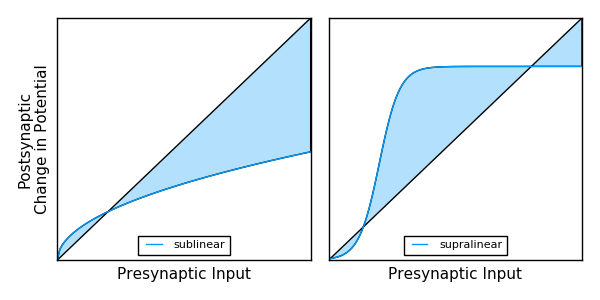
Figure C: Membrane Potential Response Shapes
This means that, depending on the structure and properties of a neuron’s dendritic tree and synaptic connectivity, membrane potential can be effected in highly nonlinear ways. Notice in Figure C how the sublinear line is the square root of input, while the supralinear line is similar to the sigmoid function. This is intentional to emphasize parallels to current deep learning methods. For more details on the sublinear and supralinear curves, see [3].
Dendritic Segments as a Key Computational Component
As hinted before, nearby synapses interact in nonlinear ways when they are active at the same time. It turns out that relative distance is not the defining characteristic; synapses are grouped into dendritic segments which are somewhat isolated from each other. Depending on the properties of the segment, as well as the location, the synaptic interaction can be linear, sublinear, or supralinear.
Remember also that dendrites have a branching, tree-like structure. One advantage is that the branch points, where the tree splits, have inhibitory properties. Thus the dendritic forks can integrate or inhibit potentials from more distal dentritic areas.
To summarize: A neuron has dendritic branches protruding from the soma. Those branches split at dendritic forks into more branches. On each branch are some number (possibly zero) of dendritic segments. A segment may contain many axodendritic synapses. Integration, inhibition, and amplification can occur at every level of the heirarchy, creating a rich and deep computation structure within a single neuron. In Julia:
abstract NeuralComponent
type Synapse <: NeuralComponent
presynaptic_neuron
presynaptic_current
end
type Segment <: NeuralComponent
synapses
end
type Branch <: NeuralComponent
segments
fork
end
type Soma
inputs::Set{NeuralComponent}
membrane_potential::Float64
end
To be continued… (when I have time)
[1] Blausen.com staff. “Blausen gallery 2014”. Wikiversity Journal of Medicine. doi: 10.15347/wjm/2014.010. ISSN 20018762.
[2] Abrahamsson, T, Cathala, L, Matsui, K, Shigemoto, R, and Digregorio, D. A. (2012). Thin dendrites of cerebellar interneurons confer sublinear synaptic integration and a gradient of short-term plasticity. Neuron. 73, 1159-1172. doi: 10.1016/j.neuron.2012.01.027
[3] Tran-Van-Minh A, Caze RD, Abrahamsson T, Cathala L, Gutkin BS and DiGregorio DA (2015) Contribution of sublinear and supralinear dendritic integration to neuronal computations. Front. Cell. Neurosci. 9:67. doi: 10.3389/fncel.2015.00067
